|
 |
 |
|
||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
Confocal Microscopy Image Galleries
The performance of any optical imaging instrument is perhaps best judged by the quality of the images produced. This gallery contains fluorescence images captured with the FV300, FV500, and FV1000 under a variety of experimental conditions and scanning modes. In several cases, the raw optical section data has been processed by deconvolution, three-dimension volume rendering, and other related techniques.
Fluorescently Labeled Cells in Culture - The culture of whole tissues and isolated cells were first undertaken in the early 1900s as a technique for investigating the behavior of animal cells in an isolated and highly controlled environment. The term tissue culture arose because most of the early cells were derived from primary tissue explants, a technique that dominated the field for over 50 years. As established cell lines emerged, the application of well-defined normal and transformed cells in biomedical investigations has become an important staple in the development of cellular and molecular biology. This laser scanning confocal microscopy image gallery explores over 25 of the most common cell lines, labeled with a variety of fluorophores using both traditional staining methods as well as immunofluorescence techniques.
Plant Tissue Autofluorescence Gallery - Autofluorescence in plant tissues is a common and useful phenomenon arising from a variety of endogenous biomolecules that absorb light in many regions of the near-ultraviolet and visible light spectrum. One of the primary contributors of plant autofluorescence is chlorophyll, but lignins, carotenes, and xanthophylls also produce a significant level of fluorescence emission when stimulated with the proper wavelengths. This digital image gallery examines natural autofluorescence in plant tissue thin sections using multiple excitation wavelengths with laser scanning confocal microscopy.
Rat Brain Tissue Sections - The rat brain has served as an excellent model for elucidating the complex anatomy and physiological mechanisms of the human brain. As a result, a significant amount of information on brain diseases, such as dementia and Parkinson's disease, has been determined from investigations using rat brains. Brain tissue has been mapped into dozens of major and hundreds of minor regions that are anatomically and functionally distinct. Individual brain cells segregate into specialized areas by expressing a wide spectrum of specific housekeeping proteins, enzymes, transporters, and receptors. This digital image gallery explores many regions of the rat brain as observed with immunofluorescence in coronal, horizontal, and sagittal thick sections using laser scanning confocal microscopy.
Drosophila Adult Brain - A three-dimensional volume rendering of confocal microscopy optical sections made from the brain of an adult fruit fly (Drosophila melanogaster) is presented by the image featured in this section. Mushroom bodies in the specimen were labeled with green fluorescent protein (GFP), and are highlighted in the green overlay. The image was contributed by Toshiro Aigaki in the Cytogenetics Department of the Tokyo Metropolitan University.
Fluorescent Protein Expression in Zebrafish Embryos - Spectral variants of the green fluorescent protein (GFP) are quite useful for double or triple labeling experiments or when targeting fluorescence expression with a particular laser system. The confocal image presented in this section is a three-dimensional volume render made from 5-micrometer serial optical sections of a Zebrafish embryo labeled with a red fluorescent protein (dsRED). The image was provided by Yasuhiro Kamei and Shunsuke Yuba of the Institute for Molecular and Cellular Biology at Osaka University in Japan.
Human Colon Crypt - Doubled labeled with the fluorochromes Alexa Fluor 488 (green fluorescence) and TO-PRO-3 (red fluorescence), the laser scanning confocal microscope image of a human colon crypt featured in this section reveals a significant amount of anatomical detail. The image was captured by Christine Anderson from the Ray White laboratory in the Huntsman Cancer Institute at the University of Utah.
Human Skin Tissue - A thick section of living human skin tissue was imaged using infrared differential interference contrast (IR DIC) with a water immersion objective at a depth of approximately 80 micrometers to produce the image illustrated in this section. The specimen was illuminated with 750 nanometer light from a near infrared laser system. This striking image reveals the potential of laser scanning confocal microscopy when coupled with traditional contrast-enhancing techniques.
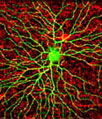
Mouse Hippocampal Neurons - Hippocampal neurons expressing a green fluorescent protein (GFP) labeled postsynaptic density protein (green) were fixed and stained with rhodamine-phalloidin to visualize the location of cytoplasmic actin filaments (red) in the image presented in this section. In dendrites, the actin filaments are concentrated in the postsynaptic sites. The image was contributed by Shigeo Okabe from the Department of Anatomy and Cell Biology at the Tokyo Medical and Dental University in Japan.
Nematodes (Caenorhabditis elegans) with Fluorescent Proteins - A nematode found in temperate regions across the Earth, Caenorhabditis elegans has been widely used in the study of genetics, development, and neurobiology. The image presented in this section features a genetically modified mutant of the nematode that expresses a version of the beta-integrin gene fused to green fluorescent protein (GFP). The image was provided by Xioping Xhu and John Plenefisch from the Department of Biology at the University of Toledo.
Rat Cerebellum Purkinje Cells - Purkinje cells in the rat cerebellum were stained with a dual fluorophore strategy to produce the beautiful confocal image presented in this section. The green fluorescent probe fluorescein isothiocyanate (FITC) was used to label vesicular gamma-aminobutyric acid transporter VGAT), while red Cy3 labeled the vesicular glutamate transporter (VGLUT1). The image was provided by Masahiko Watanabe from the Department of Anatomy at the Hokkaido University School of Medicine in Japan.
Rat Tongue Taste Buds - A thick section of rat tongue was triple stained with the nucleic acid fluorochrome DAPI, fluorescein isothiocyanate (FITC), and Texas Red to yield the striking confocal image presented in this section. Nuclei of the cells fluoresce a bright blue, while a high-affinity receptor for brain-derived neurotropic factor (TrkB) is stained green in the image. Protein gene products, distributed throughout the tissue, are stained red with the popular rhodamine derivative. The image was contributed by Shigeru Takami from the Department of Anatomy in the School of Health Science at Kyorin University in Japan.
Retina Ganglion Cell - Vividly stained with the fluorochrome Lucifer yellow, the retinal ganglion cell in the central portion of the image featured in this section is extruding a multiple of processes. Closely associated dopamine-operated amacrine cells were counterstained with Texas Red. The confocal image was provided by Professor Shigetada Nakanishi of the Department of Biological Sciences at the Kyoto University Faculty of Medicine in Japan.
Swallowtail Butterfly Visual Interneurons - Visual interneurons obtained from the beautiful swallowtail butterfly were injected with the fluorochrome Lucifer yellow in order to capture the image illustrated in this section. The specimen was imaged with extended focus in 100-micrometer serial sections through the 383-micrometer axial range of the specimen and displayed in an overlapping pseudo coloring scheme. The image was provided by Mituyo Kinoshita and Kentaro Arikawa from the Laboratory of Neuroethology in the Graduate School of Integrated Science at the Yokohama City University in Japan.
Zinnia elegans Mesophyll Cells - Isolated mesophyll cells from the flowering plant Zinnia elegans were multiply stained and reconstructed using volume rendering techniques to produce the image illustrated in this section. The image was provided by Keisuke Obara and Hiroo Fukuda from the Department of Biological Sciences in the Graduate School of Science at the University of Tokyo in Japan.