Specimen Preparation Protocols
Section Overview:
Confocal microscopy was becoming more than just a novelty in the early 1980s due to the upswing in applications of widefield fluorescence to investigate cellular architecture and function. As immunofluorescence techniques, as well as the staining of subcellular structures using synthetic fluorophores, became widely practiced in the late 1970s, microscopists grew increasingly frustrated with their inability to distinguish or record fine detail in widefield instruments due to interference by fluorescence emission occurring above and below the focal plane. Today, confocal microscopy, when coupled to the application of new advanced synthetic fluorophores, fluorescent proteins, and immunofluorescence reagents, is one of the most sophisticated methods available to probe sub-cellular structure. The protocols described in this section address the specimen preparation techniques using synthetic fluorophores coupled to immunofluorescence that are necessary to investigate fixed adherent cells and tissue cryosections using widefield and confocal fluorescence microscopy.
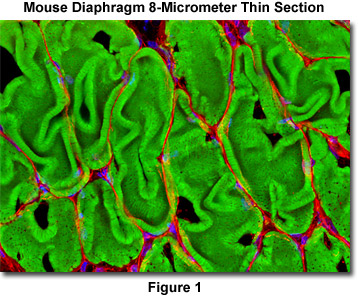
Presented in Figure 1 is a laser scanning confocal image revealing the extensive filamentous actin network present in the smooth muscle tissue of a thin (8-micrometer) cryosection of rat diaphragm. The tissue cryosection was labeled with a cocktail containing Alexa Fluor 488 conjugated to phalloidin (staining actin) and Texas Red-X conjugated to wheat germ agglutinin (targeting lectins). In addition, nuclei in the specimen were counterstained with Hoechst 33342. Images were recorded in grayscale with an Olympus FluoView FV1000 coupled to a BX-81 inverted microscope using Argon-ion (488 nanometer line), violet diode (405 nanometers), and green helium-neon (543 nanometers) lasers. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
Staining Protocols
-
Triple-Staining Adherent Cells with MitoTracker, Phalloidin (or Phallacidin), and Nuclear Dyes
The vitality and physical properties of adherent cells grown on coverslips in Petri dishes can be determined using a combination of fluorescent stains. This protocol details a generalized procedure for staining a variety of cell types.
-
Staining Adherent Cells with Intermediate Filament Primary Antibodies and Synthetic Fluorophores
Fibroblast and epithelial cell lines derived from humans and laboratory animals produce brightly colored fluorescent specimens highlighting specific proteins in the intermediate filament network when stained.
-
Staining Cells and Tissue Cryosections with Tubulin Primary Antibodies, Phallotoxins, and Synthetic Fluorophores
Cell lines and mammalian tissue sections derived from humans and laboratory animals, including intestine, kidney, testes, muscle, liver, and the lungs, produce brightly colored fluorescent specimens.
-
Staining Adherent Cells with Cytokeratin Primary Antibodies and Synthetic Fluorophores
Epithelial cell lines derived from humans and laboratory animals produce brightly colored fluorescent specimens detailing the cytokeratin intermediate filament network when stained with keratin antibodies.
-
Triple-Staining Tissue Cryosections with Wheat Germ Agglutinin, Phalloidin, and Nuclear Dyes
A majority of the common tissue sections derived from laboratory animals, including intestine, kidney, testes, muscle, liver, and the lungs, produce brightly colored fluorescent specimens detailing a wide variety of anatomical features when stained.
-
Immunofluorescence with Brain Tissue Cryosections
Among the antibody targets often labeled in brain tissue sections are glial fibrillary acidic protein (GFAP), neuronal and axonal neurofilaments, class III beta-tubulin, microtubule-associated proteins, blood-brain barrier proteins, and antigens associated with specific diseases.
-
Immunofluorescence with Brain Tissue Floating Cryosections
Indirect immunofluorescence is a technique that enables the visualization of specific targets using a combination of primary and secondary antibodies. This protocol details a procedure for staining frozen brain floating cryosections greater than 30 micrometers in thickness.
