Green Fluorescent Protein (GFP)
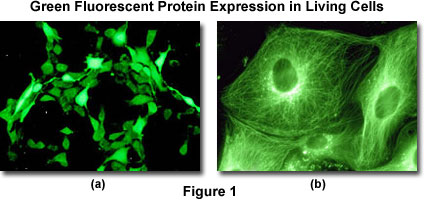
The green fluorescent protein (GFP) and its spectral variants (yellow, YFP; cyan, CFP; blue, BFP; and red, dsRFP) are in rapidly becoming important investigational tools in the various disciplines associated with the life sciences including medicine and biology. A quick search of the Internet or the recent literature reveals dozens or more reports each month. The green fluorescent protein was originally isolated from the jellyfish and is a specialized protein that emits fluorescence when exposed to excitation light. Its primary importance for current research lies in the ability of the purified jellyfish GFP gene to express the fluorescent protein in other living organisms. When a non-specific fluorescent protein (or a variant chimera) gene is introduced into a tissue culture line, the entire cellular cytoplasm will ultimately emit a green fluorescence as the protein is distributed throughout the cell (illustrated in Figure 1(a)).
The green fluorescent protein consists of 238 amino acids arranged in a helical basket motif. Three amino acids form an annular structure inside the basket by special bonding; it is this part that emits fluorescence.
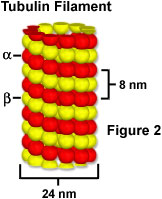
It is possible to make a fusion gene combining the green fluorescent protein gene product with another protein (for example alpha-tubulin, which is one of proteins comprising a microtubule, as illustrated in Figure 2), and to express the chimera inside a cell. When such a fusion gene is introduced into a cell, the alpha-tubulin tagged with fluorescent protein is produced inside the cell and subsequently incorporated into the microtubule to form a tubular structure that emits green fluorescence (illustrated in Figure 1(b)). This technique makes it possible to visualize the localization of a specific gene product (in effect, a protein) inside living cells. It enables the researcher to observe the localization of proteins in the living state while the fluorescence antibody technique fixes the cell (causing its death).
The following steps demonstrate how a fused chimeric protein is expressed in a host cell line.
Preparation of a Fusion Gene
The alpha-tubulin gene is prepared from human brain complementary DNA (cDNA) libraries that can be purchased from commercial sources. A cDNA library is synthesized by templating the sequences of mRNA that are expressed in the cells. This cDNA is integrated in annular (non-chromosomal) DNA, termed plasmids, for storage and distribution. Some of the plasmids integrate the alpha-tubulin gene, and these plasmids are used as templates for amplifying alpha-tubulin by means of polymerase chain reaction (PCR) techniques. At present, many GFP vectors containing fluorescent proteins fused to specific cellular targets can be purchased from BD Biosciences Clontech Division, in Palo Alto, California or Qbiogene, Inc. (Quantum Biotechnologies Inc.), in Illkirch Cedex, France. Selection of the fluorescent protein vector depends on whether the gene of interest is linked at the 3' or 5' end of the fluorescent gene product sequence.
Introduction Fusion Genes into the Cell
To express the fluorescent protein-tubulin fusion gene product in the cell, it is first necessary to introduce the fusion gene as a plasmid vector into the cell using one of the following techniques.
- Microinjection - Plasmids can be injected directly into the cell nucleus. This method is unsuitable for expressing genes in large numbers of cells, but is used in specialized cases or when particular cells are the targets.
- Lipofectamine transfection - When a mixture of positively charged lipids and neutral lipids is blended with DNA, a complex is formed by an electrostatic interactions and taken up by the cell through phagocytosis or membrane fusion. Lipofectamine transfection has high efficiency and low toxicity.
- Calcium phosphate precipitation - When calcium phosphate is mixed with DNA, a complex is precipitated that can subsequently be employed to transfect susceptible cells. Calcium phosphate precipitation is one of the simplest techniques for conducting transfections using plasmid vector DNA. When this precipitate is applied to the cell, part of it is caught inside the nucleus.
- Electroporation - A high-voltage pulse is applied to the cell to create pores in the external membrane. Vector DNA can enter through the pores to express its gene products.
The fusion protein will be expressed several hours after the gene introduction, but the culture should be continued for about two days until the fluorescent tubulin is fully integrated in the microtubule network.
It is also possible to apply double staining with two proteins emitting different fluorescent colors. Besides GFP, the available fluorescent proteins include BFP (blue fluorescent protein), CFP (cyan fluorescent protein) and YFP (yellow fluorescent protein). Recently, a red fluorescent protein (dsRFP) has been introduced by Clontech.
