Chameleons: Calcium Ion Probes
Cameleons are a new class of indicators for calcium ion concentrations in living cells, which operate through a conformational change that results in fluorescence resonance energy transfer (FRET) in the presence of calcium ions. In the past, fluorescent probes, such as Fura-2, Indo-1, and Fluo-3 were very popular for measuring fluctuations in calcium ion concentrations within living cells. In 1997, Dr. Atsushi Miyawaki (of the Riken Brain Science Institute in Wako, Japan) developed a novel probe for calcium ion measurement. This probe consists of an artificial protein modified from green fluorescent protein (GFP), and was named Cameleon (after the chameleon reptile, but dropping the "h" from the name). The cameleon molecular structure is modeled as a fusion product between two fluorescent proteins (having differing excitation and emission characteristics), calmodulin (CaM), and the calmodulin-binding domain of myosin light chain kinase (M13). Calmodulin is capable of binding with free calcium ions and the M13 chain can bind with calmodulin after it has bound the calcium ions. The genes of these four proteins are joined linearly, and the fusion genes are expressed in a variety of cells.
The cameleon chimera is remarkable for its practical applications in genetic engineering and FRET, a biophysical technique for measuring the distance between two closely spaced molecules. A pair of fluorescent proteins for FRET is selected according to their characteristic absorption and emission spectra. The excitation energy of one fluorophore (the donor) is transferred to another (the acceptor) by dipolar interactions, without donor fluorescence emission. The donor emission and acceptor absorption spectra must overlap for FRET to occur. In addition, energy transfer takes place only when the two fluorophores are within a few nanometers of each other. Fluorescent protein pairs, particular a cyan and yellow or blue and green (CFP/YFP and BFP/GFP) pair are generally chosen for FRET experiments because of their spectral characteristics.
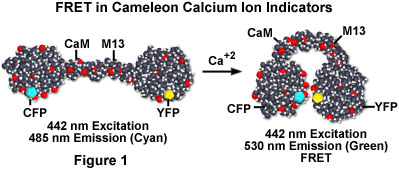
The cameleon molecule consists of four domains (see Figure 1). Fluorescent proteins joined at both ends stand away from each other in the absence of calcium ion, but in the presence of calcium ions, the activated CaM linker wraps around the M13 protein. The tertiary structure of the cameleon chimera is then altered to bring the two fluorescent protein moieties with range of energy transfer. In a low calcium concentration environment, the cameleon molecule is unfolded and CFP fluorescence occurs when the molecule is excited with 442-nanometer light. However, when the calcium ion concentration increases, CFP fluorescence decreases and YFP fluorescence increases due to resonance energy transfer.
The binding of calcium by the calmodulin moiety of cameleon produces a conformational change of the entire molecule, which positions the two fluorescent proteins into close spatial proximity. In this conformation, dipolar energy transfer by the excited CFP protein stimulates the YFP to produce secondary fluorescence at 530 nanometers. The cameleon design represents the first genetically encoded calcium sensor, and offers the opportunity to monitor cellular structural and physiological dynamics in a relatively non-invasive manner. Intracellular calcium ion concentration can be determined by fluorescence ratio imaging as the ratio of the changes in two types of cameleon molecules according to the intracellular concentration of calcium ion. When the cameleon molecule has been excited at the 442 nanometers (using a line from the helium-cadmium laser), its fluorescence can be detected simultaneously at 485 and 530 nanometers using confocal laser scanning microscopy.

A typical calcium ion monitoring experiment by cameleon is presented in Figure 2. In this experiment, the cameleon chimera was similar to the CFP/YFP variant described above. The two genes were expressed simultaneously in HeLa cells transfected by the lipid technique. In Figure 2(a), the calcium levels without the presence of stimulants or depressants are illustrated. Stimulation by 0.1 millimolar histamine produces a dramatic increase in the concentration of calcium (Figure 2(b)), while addition of 0.1 millimolar cyproheptadine elicits an antagonistic action (Figure 2(c)) that results in reduction of calcium levels.
