Spontaneous and Stimulated Processes
One of the most important concepts necessary in understanding laser operation is the fact that quantization of energy in the atom results in discrete energy levels. In addition, transitions from one energy level to another must be possible in order for light emission to occur, and these transitions include both spontaneous and stimulated emission. This tutorial explores the concepts of spontaneous emission, as well as stimulated absorption and emission.
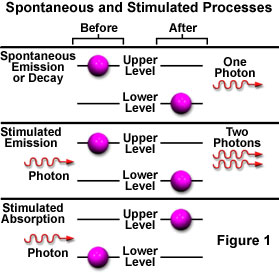
The purple spheres represent electrons in two energy levels, arbitrarily termed a Lower Level and an Upper Level. When the tutorial initializes, the electrons are randomly distributed between these two levels and can undergo transitions from one level to the other by either absorbing or emitting light. Lower level electrons can only absorb light by stimulated absorption in order to be promoted into the upper level. Once an electron resides in the upper level, it can either spontaneously emit a light wave and drop to the lower level (spontaneous emission) or it can be stimulated by a light wave to emit a second wave and return to the lower level (stimulated emission).
If an atom or molecule lies in an energy state that is higher than the lowest, or ground level state, it can spontaneously drop to a lower level without any outside stimulation. One possible result of dropping to a reduced energy state is the release of the excess energy (equaling the difference in the two energy levels) as a photon of light. Excited atoms or molecules have a characteristic spontaneous emission time, which is the average time that they remain in the excited higher energy state before they drop to a lower energy level and emit a photon. The emission time is an important factor in producing stimulated emission, the second type proposed by Einstein.
While in the excited state, if the atom is illuminated with an incoming photon having exactly the same energy as the transition that would spontaneously occur, the atom may be stimulated by the incoming photon to return to the lower state and simultaneously emit a photon at that same transition energy. A single photon interacting with an excited atom can therefore result in two photons being emitted. If the emitted photons are viewed as a wave, the stimulated emission will oscillate at the incoming light's frequency and be in phase (coherent), resulting in amplification of the original light wave's intensity. Figure 3 illustrates spontaneous (a) and stimulated (b) emission with the two coherent waves that result from the latter case.
The primary problem in achieving stimulated laser emission is that, under normal conditions of thermodynamic equilibrium, the population, or number of atoms or molecules at each energy level, is not favorable to stimulated emission. Because of the tendency of atoms and molecules to spontaneously drop to lower energy levels, the number at each energy level decreases as the energy increases. In fact, under normal conditions, for a transition energy corresponding to a typical optical wavelength (on the order of 1 electron-volt), the ratio of the number of atoms or molecules in the higher energy state to the number in the lower ground state is perhaps 10 E+17. In other words, virtually all of the atoms or molecules are in the ground state for a visible-wavelength energy transition.
The reason that stimulated emission is difficult to achieve becomes apparent when considering the likely events surrounding the decay of an electron from an exited state with the subsequent and spontaneous emission of light. The emitted light could easily stimulate emission from another exited atom, but so few are available that the emission more likely will first encounter an atom in the ground state, and will be absorbed instead. Because the number of atoms in an exited state is so miniscule in relation to the number in the ground state, the emitted photon has a much greater probability of being absorbed, rendering stimulated emission insignificant when compared to spontaneous emission (at thermodynamic equilibrium).
The mechanism by which stimulated emission can be made to dominate is to have more atoms in the excited state than in the lower energy state, so that emitted photons are more likely to stimulate emission than to be absorbed. Because this condition is the inverse of the normal equilibrium situation, it is termed a population inversion. As long as there are more atoms in the upper energy level than in the lower, stimulated emission can dominate, and a cascade of photons results. The first emitted photon will stimulate the emission of more photons, these subsequently stimulate the emission of still more, and so on. The resulting cascade of photons grows, resulting in the amplification of emitted light. If the population inversion terminates (the ground state population becomes dominant), spontaneous emission will again become the favored process.
