Optical Sectioning in DIC Microscopy
The ability to image a specimen in differential interference contrast (DIC) microscopy with large condenser and objective numerical apertures enables the creation of optical sections from a focused image that are remarkably shallow. Without the disturbance of halos and distracting intensity fluctuations from bright regions in lateral planes removed from the focal point, the technique yields sharp images that are neatly sliced from a complex three-dimensional phase specimen. This interactive tutorial explores optical sectioning in DIC microscopy utilizing a wide spectrum of specimens that differ in thickness.
The tutorial initializes with a randomly selected image appearing in the DIC Specimen Image window and a model of an objective and specimen on the right-hand side of the window. In order to operate the tutorial, use the Objective Focus slider to focus the specimen through a range of planes starting at the upper surface and progressing through the central region to the lower surface. As the slider is translated, various image planes in the specimen will be brought into sharp focus. Simultaneously, the microscope objective model will move upward and downward with respect to the specimen to simulate the actual relationship in the microscope. A new specimen can be examined by selecting from the palette in the Choose A Specimen pull-down menu.
In all traditional forms of transmitted and reflected light microscopy, the condenser aperture iris diaphragm plays a major role in defining image contrast and resolution. Reducing the aperture size increases the depth of field and overall image sharpness while simultaneously producing enhanced contrast. However, if the diaphragm is closed too far, diffraction artifacts become apparent and resolution is sacrificed. Often, the optimum aperture setting is a compromise between accurately rendering specimen detail in sufficient contrast and retaining the resolution necessary to image minute features while avoiding diffraction artifacts.
High-performance differential interference contrast microscope optical systems produce excellent contrast with the condenser iris diaphragm partially closed (approximately 70 percent of the objective rear aperture size), but also perform superbly when the diaphragm is opened to match the objective aperture size. In order to achieve the optimum balance between resolution and contrast for optical sectioning, the microscope must be properly configured for Köhler illumination and the prism components and polarizers should be accurately aligned. High numerical aperture objectives designed for oil immersion should only be utilized to image specimen slides that have the underside oiled to the condenser.
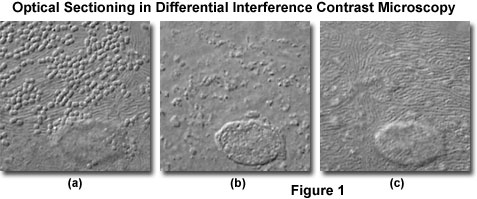
At high magnifications and numerical apertures (for example, 100x and 1.4), the depth of field in differential interference contrast approaches a limiting value of about 400 nanometers (0.40 micrometers; about 1.5 times the lateral resolution limit of the objective). Optical sections taken through the central region of a human buccal epithelial cell at high magnification (100x objective) and numerical aperture (1.30) are illustrated in Figure 1. The cell is approximately 3 micrometers thick near the nucleus, and depth of field for the configuration utilized to obtain the images in Figure 1 was approximately 0.5 micrometers. Bacteria are clearly visible on the upper surface of the cell (Figure 1(a)), as are parallel, swirling ridges in the membrane that closely resemble the texture of human fingerprints. A bulge in the membrane corresponding to the nucleus is also visible in the lower central portion of the figure. As the focal plane is shifted to the interior of the cell (Figure 1(b)), details of the nuclear structure and intracellular particles become visible. Finally, at the lower boundary where the cellular membrane rests on the surface of the microscope slide (Figure 1(c)), numerous folded ridges are revealed (similar to those observed on the upper surface).
When performing optical sectioning experiments with thicker biological specimens (especially those immersed in aqueous saline solutions), the microscopist must be alert to the possible introduction of spherical aberration produced by refractive index discontinuities at the interface between the coverslip and mounting medium. These artifacts will reduce resolution at higher penetration depths in the optical section series.
A significant amount of the current research and development of theory in differential interference contrast is focused on the striking optical sectioning characteristics of the technique. Ultimately, a quantitative estimate of the three-dimensional refractive index profile from a specimen may be achieved through computational models. In addition, current research is also directed at new models being developed for the DIC optical components and image formation in partially coherent transmitted light.
