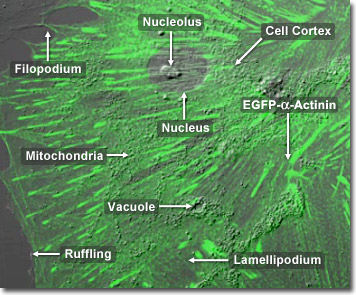
Rat Thoracic Aorta Cells with EGFP Actinin
Alpha actinins belong to the spectrin gene superfamily which represents a diverse group of cytoskeletal proteins, including the alpha and beta spectrins as well as dystrophins. Alpha actinin is an actin-binding protein with multiple roles in different cell types. In nonmuscle cells, the cytoskeletal isoform is found along microfilament bundles and adherens-type junctions, where it is involved in binding actin to the membrane. In contrast, skeletal, cardiac, and smooth muscle isoforms are localized to the Z-disc (a dark line separating two sarcomeres of muscle tissue) and similar dense bodies, where they help anchor the myofibrillar actin filaments. Alpha actinin has a molecular weight of 100,000 daltons. In the digital video presented above, embryonic rat thoracic aorta fibroblast cells (A7r5 line) are expressing a fusion of enhanced green fluorescent protein (EGFP) to human alpha-actinin.
In addition to its role in linking the actin cytoskeletal network to focal adhesions, alpha-actinin is also thought to be involved in signaling pathways. This ubiquitous protein also has a number of other molecular partners (in addition to actin), and is present in multiple subcellular regions, including cell-cell contact sites, protrusions, lamellipodia, and stress fibers.
A member of the spectrin superfamily, alpha-actinin is an actin crosslinking protein that forms antiparallel homodimers in a rod-like structure with one actin-binding domain on each side of the rod. In addition to its binding to actin, alpha-actinin also associates with a number of other cytoskeletal proteins including titin, zyxin, vinculin, and alpha-catenin, as well as cytoplasmic signaling proteins, such as phosphatidylinositol 3-kinase, Rho effector kinase, and the beta-integrins.
