Cholesteryl Acetate Time Lapse Sequences
For animals, cholesterol is essential to life, a primary component of the membrane that surrounds every cell. It is also the precursor chemical from which the body synthesizes bile acids, steroid hormones, and vitamin D derivatives. Cholesterol circulates in the bloodstream and is manufactured by the liver and several other organs.
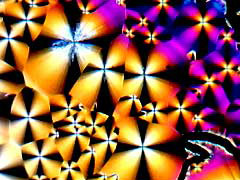
Austrian botanist Friedrich Reinitzer, in 1888, discovered that cholesteryl acetate was a flowing liquid exhibiting optical properties previously attributed only to crystals. The discovery brought into question the belief of the time that only three states of matter could exist (liquid, solid, and gas). More recently, liquid crystals such as cholesteryl acetate have come to be used in cosmetics, wrist watches, thermometers, propane tank volume indicators, video displays, and in the "mood rings" of the late 1970s fad.
Referred to as "flussige Kristallen", Reinitzer described the new phenomenon of intermediate melting points for cholesteryl acetate and the related cholesteryl benzoate, falling between those of a turbid liquid state (anisotropic) and a transparent state (isotropic). Eighty years later, Radio Corporation of America (RCA) produced the first experimental liquid crystal display (LCD) based on this fourth state of matter. Whether contained in an advanced LCD or a 1970s mood ring, the thermotropic liquid crystals twist to various degrees in response to changes in temperature. With the change in the molecular arrangement, the wavelengths of light that are reflected or absorbed by cholesteryl acetate shift, resulting in a visible color change. As a temperature change is reversed, the liquid crystal reverts to its original color before the molecular rearrangement occurred. With naturally twisted nematic liquid crystals, applying an electric current will untwist them to varying degrees, depending on the voltage. Liquid crystals or mesophases, such as cholesteryl acetate, are used in LCDs because they behave predictably in the manner in which they control light passage with the application of different electric currents. Lyotropic liquid crystals occurring in the membranes of biological cells respond in phase transition based on chemical concentrations instead of temperature, magnetic, optic, or electric cues.
Synthesis of cholesteryl acetate from cholesterol involves heating the cholesterol in a solution of acetic acid and acetic anhydride. Although synthesized in the laboratory and on a commercial scale, cholesteryl acetate exists as a naturally occurring substance as well. For example, it is found as one of the three cholesterol esters composing the outer cuticle layer lipid coating of the brown dog tick (Rhipicephalus sanguineus).
Cholesteryl Acetate Time Lapse Sequence #1
Birefringent spherulites form from the melt and merge into a nearly continuous field in this time-lapse sequence of 14 images acquired utilizing polarized light.
Cholesteryl Acetate Time Lapse Sequence #2
Crystallites form and become increasingly birefringent as the phase transition progresses and spherulites become larger in a time-lapse sequence of 19 images. The higher order colors seen in the upper right-hand corner of the field are most likely due to thickness variation in the specimen.
Cholesteryl Acetate Time Lapse Sequence #3
This 25-image time-lapse sequence illustrates the progressive phase change in cholesteryl acetate from the melt to the spherulitic state exhibiting beautiful colors of birefringence.
Cholesteryl Acetate Time Lapse Sequence #4
A 25-image time-lapse sequence begins with a single spherulitic crystallite, which expands dramatically to merge with other spherulites of widely varying size.
Cholesteryl Acetate Time Lapse Sequence #5
Beginning with a single small crystallization site, this sequence of 30 images progresses to show the formation of several large crystallites, followed by the filling of the remaining viewfield by much smaller spherulites.
Cholesteryl Acetate Time Lapse Sequence #6
A change in state from the melt to a confluent field of spherulites is illustrated in a 26-image sequence.
Cholesteryl Acetate Time Lapse Sequence #7
Several fairly large crystallites form and begin to dominate the viewfield before an increased nucleation rate allows a large number of smaller spherulites to rapidly fill the remaining volume in this time-lapse sequence of 28 images.
Cholesteryl Acetate Time Lapse Sequence #8
An 18-image sequence illustrates progressive crystallization throughout the viewfield area, but with strong birefringence seen only in one-half of the field.
Cholesteryl Acetate Time Lapse Sequence #9
The change of state illustrated in this sequence of 19 images involves predominantly larger-size crystallites, which fill the viewfield before more rapid nucleation can produce smaller spherulites.
Cholesteryl Acetate Time Lapse Sequence #10
A 30-image sequence illustrates an unusual crystallization pattern taking place at one edge of the specimen slide, where the spherulitic crystallites are restricted from forming in their typical shapes.
Cholesteryl Acetate Time Lapse Sequence #11
An atypical crystallization pattern is shown in a sequence of 28 images, in which the formation of the spherulitic phase is altered at the edge of the melt on the microscope slide, possibly by excess surface tension at the interface.
Cholesteryl Acetate Time Lapse Sequence #12
Spherulites appear to grow in an unusual fashion adjacent to several melt boundaries that have formed near the edge of the specimen mount in this time-lapse sequence of 25 images.
Cholesteryl Acetate Time Lapse Sequence #13
A 21-image time-lapse sequence illustrates a relatively uniform birefringence across the viewfield as crystallization takes place.
Cholesteryl Acetate Time Lapse Sequence #14
Uniform spherulite formation across the microscope viewfield produces a nearly monochromatic sequence of 18 time-lapse images showing very low-order birefringence.
Cholesteryl Acetate Time Lapse Sequence #15
Some moderate-order birefringence in the spherulitic phase is exhibited in a time-lapse sequence of 30 images.
