|
 |
 |
|
||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
Specialized Confocal Techniques
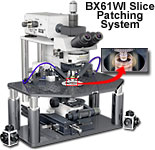
The Olympus FluoViewTM confocal microscope systems can be easily configured with a variety of optical and mechanical accessories for specialized techniques, such as slice patch clamping, differential interference contrast, and observations using infrared and ultraviolet illumination.
Olympus continues to pursue the development of advanced accessories designed to meet the increasing sophistication of demanding applications that expand the possibilities of confocal microscopy. From slice patching systems and high numerical aperture water immersion objectives for live-cell imaging to specialized software for ratio imaging and time-lapse analysis, the ongoing development of FluoViewTM laser systems, scanning units, and software packages remains a top priority for Olympus scientists and engineers.
Superior Slice Patching System
Using the FV300 or FV500 in combination with the unique fixed stage and nosepiece focusing BX61WI upright microscope provides a highly effective system for slice patching. This unique setup has a small footprint that ensures ample room in the limited cage. The microscope remote control function minimizes the danger of accidentally disturbing the delicate experimental configuration. Olympus also offers highly corrected non-cover glass long working distance water immersion objectives and optional movers that translate the entire microscope system while the specimen and micromanipulators remain in fixed position.
Water Immersion LUMPLFL Objectives

The 40x water immersion objective in this series has a 3.3-millimeter working distance and an extremely fine tip, which is suitable for micromanipulation using a fixed stage upright microscope. The objective has a large numerical aperture (0.80) and is also ideal for confocal observations. When coupled to the BX61WI (fixed stage and nosepiece focusing upright microscope) with water immersion objectives, confocal imaging can be used to monitor time-lapse fluorescence changes in thick specimens, such as brain slices.
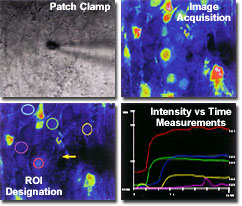
Highly Precise Time-Lapse Analysis
The Olympus FluoView'sTM wide dynamic range of 12-bit or 4096 gray levels yields enough precision to detect even the slightest specimen changes. The user can designate multiple regions of interest (ROIs) by using the convenient software drawing tools. The intensity or the ratio of intensities can be analyzed with the intuitive FluoViewTM software graphical user interface. The confocal images can be analyzed with respect to multiple parameters, including length, distance, size, integration area and average fluorescence intensity measurements, as well as intensity changes over time and distance. All quantitative data recorded by the microscope can be stored in a spreadsheet format for subsequent analysis in popular software programs.
Infrared Observations

With a near-infrared diode laser attached to the scanning head of the FluoViewTM confocal microscope, differential interference contrast (DIC) observations of living cells and tissues can be coupled to fluorescence analysis at depths exceeding 30-40 micrometers. Deeper parts of the tissue are observed far better using near-infrared laser illumination, which features a high transmission rate and produces less photo damage to living tissue. For example, the DIC image of human skin tissue on the immediate right was recorded using a 543-nanometer helium-neon laser at a depth of approximately 80 micrometers. In contrast, the image on the far right was produced with a 750-nanometer near-infrared laser in the same field of view and identical instrumental parameters. Note the superior resolution and sharpness in the image produced with the near-infrared laser.
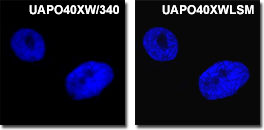
Ultraviolet Observations
Olympus' new and unique ultraviolet-corrected objectives enable superior confocal imaging of fluorophores excited below 400 nanometers when they are combined with standard visible light fluorescent probes. The FV500 simultaneously captures up to 5 channels, with 4 channels dedicated to fluorescence and one channel for brightfield and DIC. The living PtK2 kangaroo rat cell nuclei presented in the figure on the left were stained with DAPI and imaged with either a standard ultraviolet (far left; the UVAPO40xW/340 objective) or high-performance ultraviolet water immersion Olympus objective (the UVAPO40xWLSM). Note the increased contrast and resolution in the images provided by the specially corrected objective.
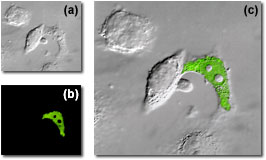
Differential Interference Contrast Observations
The combination of DIC (available in the FluoViewTM with a dedicated photomultiplier) with fluorescence observations can be employed to localize probes to specific sub-cellular components. This is a powerful technique that is finding an increasing number of applications in the biomedical sciences. As an example, the subcellular localization of messenger RNA expression for human immunodeficiency virus (HIV) in living HeLa cells is illustrated in the figure on the right. In frame (a), the cells are imaged with DIC alone, while frame (b) shows the fluorescein (FITC) labeled RNA fluorescence image generated by an argon-ion laser photomultiplier channel. When the images are overlaid (frame (c)), the distribution of messenger RNA in the infected cell and be readily evaluated.