|
 |
 |
|
||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
Fluorescence Resonance Energy Transfer (FRET)

The Olympus FluoViewTM provides hardware and software support to optimize the environment for fluorescence resonance energy transfer (FRET) investigations.
The precise location and nature of the interactions between specific molecular species in living cells is of major interest in many areas of biological research, but investigations are often hampered by the limited resolution of the instruments employed to examine these phenomena. Conventional widefield fluorescence microscopy enables localization of fluorescently labeled molecules within the optical spatial resolution limits defined by the Rayleigh criterion, approximately 200 nanometers (0.2 micrometer). However, in order to understand the physical interactions between protein partners involved in a typical biomolecular process, the relative proximity of the molecules must be determined more precisely than diffraction-limited traditional optical imaging methods permit. The technique of fluorescence resonance energy transfer (more commonly referred to by the acronym FRET), when applied to confocal microscopy, permits determination of the approach between two molecules within several nanometers, a distance sufficiently close for molecular interactions to occur.

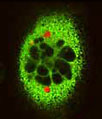
The helium-cadmium (HeCd) laser system option for the Olympus FluoViewTM confocal microscopes can provide a useful avenue for resonance energy transfer experiments using the popular cyan and yellow fluorescent protein (CFP and YFP) combination. The 442-nanometer spectral line from the helium-cadmium laser excites CFP very efficiently with virtually no excitation of the YFP fluorophore at the same wavelength. In addition, the Olympus high-performance objectives designed specifically for laser scanning confocal microscopy, PLAPO40xWLSM and PLAPO60xWLSM, are precisely corrected in this wavelength region, and ensure the highest measuring reliability. Note that for observation of energy transfer between CFP and YFP, simultaneous excitation must be conducted at 442 and 515 nanometers. An example of FRET in HeLa cells is provided in the image to the right for ratio changes when cameleon is manifested on the HeLa cell and stimulated by histamine, and then inhibited by cyproheptadine. The experiment was conducted on a FluoViewTM FV300 with a helium-cadmium laser (courtesy of Dr. Miyawaki Atsushi).

Separate channels utilized in measuring the calcium ion concentration in a living HeLa cell using a cameleon (split type) indicator are presented above. Energy transfer between CFP and YFP is proportional to bound calcium. The time series illustrates the increase of calcium ion density produced by stimulation of histamine and the effect of blocking by proheputajin.
Ratio Imaging to Analyze Dual-Wavelength Images

Using time course software, the ratio image can be continuously displayed in pseudo color. At the same time, the intensity of each channel can be monitored graphically (see the CFP/YFP experiment illustrated above). The analyzing processes are presented as an instinctive flow chart. Optional time course software (TIEMPO) is also available to provide assistance in these investigations.
Input/Output of External Trigger Signal
The optional time course software provides control over the input/output trigger signal through an intuitive graphical user interface. This software package is suitable for combined experiments, such as those that involve patch clamping.