|
 |
 |
|
||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
Confocal Microscopy Image Gallery
Rat Brain Tissue Sections
Third Ventricle
The ventricular system of the brain is comprised of several cavities and the narrow channels that connect them to one another. Contained within the system is the watery material known as cerebrospinal fluid, which provides mechanical protection to the brain and is central to the removal of potentially toxic metabolites from the organ.

Cerebrospinal fluid is primarily secreted by the choroid plexus located in the lateral ventricles (formerly known as the first and second ventricles), which are paired in the cerebral hemispheres. From the lateral ventricles the fluid drains into the third ventricle via the interventricular foramina. The third ventricle, which is positioned between the two hemispheres of the diencephalon, is linked to the fourth ventricle by the aqueduct of Sylvius, and through this channel the cerebrospinal fluid enters this final ventricle located between the pons and the cerebellum.
The third ventricle is a narrow cavity positioned vertically in the brain. The anterior, posterior, dorsolateral, and ventrolateral walls that demarcate the third ventricle are formed by the lamina terminalis, the epithalamus, the thalamus, and the hypothalamus, respectively. A horizontal groove called the hypothalamic sulcus marks the division between the sections of the walls comprised of the thalamus and the hypothalamus. In 70 to 80 percent of humans, the two thalami of the brain are connected across the third ventricle by the massa intermedia, a band of gray matter. Several depressions, or recesses, are located along the cavity, including the infundibular, optic, mammillary, and the pineal recesses.
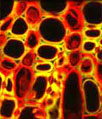
Glial fibrillary acidic protein (GFAP) and vimentin are both members of the class III intermediate filament protein family that were visualized in the rat hypothalamus section illustrated above, which prominently features the third ventricle. Before being treated with a cocktail of rabbit anti-GFAP and mouse anti-vimentin primary antibodies, the specimen was fixed, permeabilized, and blocked with 10-percent normal goat serum. The primary targets were visualized with goat anti-rabbit and anti-mouse secondary antibodies conjugated to Alexa Fluor 568 and Alexa Fluor 488, respectively. Cell nuclei were detected with DRAQ5 (pseudocolored blue), a far-red fluorescent DNA probe. Images were recorded with a 20x objective using a zoom factor of 1.4 and sequential scanning with the 488-nanometer spectral line of an argon-ion laser, the 543-nanometer line from a green helium-neon laser, and the 633-nanometer line of a red helium-neon laser. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles unless otherwise noted above.
Additional Confocal Images of Rat Brain Third Ventricle Tissue Sections
Targeting Vimentin and Glial Fibrillary Acidic Protein in Coronal Rat Brain Sections - In a double immunofluorescence experiment, a coronal rat brain section featuring the third ventricle was labeled with mouse anti-vimentin and rabbit anti-GFAP primary antibodies followed by goat anti-mouse and anti-rabbit secondary antibodies conjugated to Alexa Fluor probes (488 and 568, respectively). In order to counterstain cell nuclei, the specimen was subsequently treated with DRAQ5.
Rat Brain Tissue Triple Labeled with Alexa Fluor 488, Alexa Fluor 568, and DRAQ5 - The intermediate filament protein vimentin was targeted in this rat brain tissue section, which features the hypothalamus and the third ventricle, with mouse anti-vimentin monoclonal antibodies followed by goat anti-mouse secondary antibodies conjugated to Alexa Fluor 488. In addition, glial fibrillary acidic protein was immunofluorescently labeled with rabbit anti-GFAP primary antibodies visualized with goat anti-rabbit secondaries conjugated to Alexa Fluor 568. DRAQ5 (pseudocolored cyan) was subsequently employed as a nuclear counterstain.
Contributing Authors
Nathan S. Claxton, Shannon H. Neaves, and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.