 |
 |
 |
|
||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
Applications in Confocal Microscopy
Fluorescence Lifetime Imaging Microscopy (FLIM)
Multi-color staining with fluorescent dyes is actively used for observing the distribution of biological materials (such as proteins, lipids, nucleic acids, and ions) in the field of tissue and cell research. The detection technology for fluorescence observation has advanced to a level at which a single fluorescent dye molecule can be detected under the best of circumstances. This section reviews several of the important aspects of fluorescence lifetime imaging microscopy (FLIM), a new fluorescence microscopy technology. In addition to multi-color staining, fluorescence lifetime imaging can also be utilized to visualize the factors that affect the fluorescence lifetime properties of the dye molecule, that is, the state of the environment around the molecule.
Wavelength Spectroscopy
Conventional fluorescence microscopy makes use of the color properties of fluorescent dyes, that is, identification is based on differences in fluorescence spectral characteristics between dyes. With this technique, five or six dyes in the wavelength range from ultra violet to near infrared can be used simultaneously under microscopy with no confusion between colors.
Lifetime Spectroscopy
Each fluorescent dye has its own lifetime in the excited state. By detecting differences in lifetime, it is possible to distinguish even dyes having the same fluorescent color as well as to identify autofluorescence. Furthermore, high signal-to-noise images can be obtained by using a probe with very long lifetime compared to that of the fluorescent dyes normally used. For instance, platinum coproporphyrin has a lifetime of millisecond order while the lifetimes of ordinary fluorescent dyes are of nanosecond order. Such relatively long-lived fluorescent dyes will soon be used as probes for DNA detection on chips.
Fluorescence lifetime imaging also makes it possible to obtain information on the molecules while observing a living cell. The factors affecting the fluorescence lifetime include ion intensity, hydrophobic properties, oxygen concentration, molecular binding, and molecular interaction by energy transfer when two proteins approach each other. Lifetime is, however, independent of dye concentration, photobleaching, light scattering and excitation light intensity. Therefore, fluorescence lifetime imaging allows us to perform accurate ion concentration measurement and Fluorescence Resonance Energy Transfer (FRET) analysis.
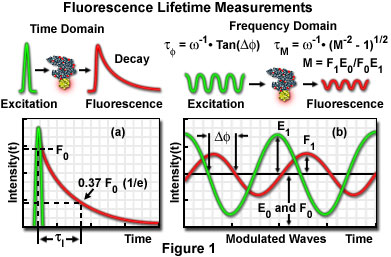
There are two methods of fluorescence lifetime imaging: the time-domain method and the frequency-domain method.
- Time-domain FLIM - In some cases of delay after excitation by a pulse laser, the fluorescence image can be obtained by the gate operation of the image intensifier. The lifetime is measured in nanoseconds by a laser with a pulse duration of a few hundred picoseconds and a nanosecond-level shutter because the lifetime of an excitation state is usually 1 to 20 nanoseconds. A high-speed gate image intensifier is commercially available from Hamamatsu Photonics K.K. (Hamamatsu, Japan). The fluorescence lifetime at each pixel can also be obtained by measuring while varying the delay time until a gate opens. Fluorescence lifetime images are shown in pseudocolor according to their lifetimes.
- Frequency-domain FLIM - Fluorescence lifetime is calculated by measuring the phase shift of fluorescence and the reduction in its amplitude using a detector with a gain modulator when the laser used as the excitation light source is modulated (1 to 200 megahertz). The measurement may be taken either by laser scanning (photomultiplier) or using a charge-coupled device (CCD).
Applications
The environment surrounding the probe is detected based on the fact that the fluorescence lifetime is sensitive to hydrogen ion concentration (pH), oxygen, and calcium ion concentrations. The binding or the interaction between molecules can also be measured in combination with FRET.
Calcium Ion Concentration Imaging
When the calcium ion binds to a fluorescent probe such as Fura-2, Fluo-3 or Calcium Green, both the fluorescence lifetime and the fluorescence intensity change. The conventional procedure for ion concentration measurement focuses on the change in intensity. According to the change of the calcium ion concentration, the ratio of dyes between bound and unbound calcium ion changes, and this subsequently leads to a change in the fluorescence lifetime of the measuring spot in the specimen. In addition to the calcium ion probe, this technique is also applicable to the measurement of pH and other ions such as sodium ion and magnesium ion.
Fluorescence Resonance Energy Transfer (FRET)
Research is currently being conducted on FRET by green fluorescent protein (GFP) variants (GFP with a different fluorescence color). FRET makes it possible to measure the interactions (association or dissociation) between two proteins that are labeled with a pair of fluorescence dyes. A donor fluorescent dye has shorter excitation/emission wavelengths that provide energy to an acceptor fluorescence dye. The lifetime of the excitation state of the donor dye is variable depending on whether or not the acceptor (the dye receiving the energy) exists. Measurement based on lifetime permits better quantification because it is not necessary to consider the overlap of fluorescence during detection.
Clinical Imaging
As some tissue and cytodiagnostic specimens have strong autofluorescence, the use of probes with long lifetimes (up to milliseconds) has been attempted. Long-lifetime probes are also useful in Fluorescence in situ hybridization (FISH) because the number of colors that can be used simultaneously is limited with this technique. The hydrogen ion concentration in blood, as well as the oxygen and carbon dioxide pressures, have already been measured based on fluorescence lifetime, although such measurements are still not possible under microscopy.
Internet Resources
- Center for Fluorescence Spectroscopy - Hosted by Professor Joseph R. Lakowicz at the University of Maryland, this website is an excellent resource for information about fluorescence lifetime imaging and other aspects of fluorescence spectroscopy and microscopy.
- Kentech Instruments - Kentech manufactures high voltage solid state pulse generators and optical gated imaging systems for fluorescence lifetime imaging.
- Hamamatsu Photonics - In addition to their excellent lineup of digital camera systems, Hamamatsu also manufactures photomultipliers, avalanche photodiodes, and high-speed gate image intensifiers.
- PRS BioSciences - Specializing in biological fluorescence microscopy, PRS BioSciences manufactures an aftermarket time-gated system that can be adapted to many research microscopes.
